E t O H. Rank the following compounds in order of decreasing acid strength using periodic trends.

Rank The Following Compounds In Decreasing Order Of Acidity Image Showing Three Organic Compounds A B And C 1 B A C 2 B C A 3 C
Fromstrongest to weakest of the phenol group and briefly explain your answer.

. We are asked to rank the following compounds in order of decreasing acidity. IV Carboxylic acid OC-O-H Acidic due to electronegative O and resonance due to pi bond. HF H2O NH3 CH4ExplanationMore electronegative elements often form better acids when bonded to hydrogen.
Can you explain this answer. Hence the order of acid strength is 3142. Rank the following compounds in order of decreasing acidity O OH O CI H ch CH3 H3C I I CI OH CH3 CI OH H H A B.
Rank the following compounds in order of decreasing acidity putting the most acidic first. A IV II III I B IV III II I C III IV II I D III IV I II. Find the hydrogen and analyze where it is attached.
See the answer See the answer done loading. 4 Rank the following compounds in order of decreasing acidity putting the most acidic first. 10 pts O O O H N O OH ClH 2 C OH O 1 3 5 4 2 5.
EduRev Class 11 Question is disucussed on EduRev Study Group by 2362 Class 11 Students. Since presence of NO 2. You must be signed in to discuss.
Rank the acids from strongest to weakest. Rabo the acids shown in decreasing strongest to weakest order of acidity. Now pka -log ka where ka is the acid dissociation constant.
Rank the following compounds in order of decreasing acidity putting the most acidic first. A IV II III I C III IV II I B IV III II I D III IV I II Page 4 IV III II I Which of the following bases are strong enough to significantly deprotonate ethanol. View Available Hint s che down Reset Help ises ases BeH HI HCI H20 tion is the vity Greatest.
Rank the following compounds in decreasing order most to least of acidity. In the given compounds it is the alpha-hydrogen which contributes to the acidity. Arrange the following compounds in order of decreasing acidity.
Rank the acids from strongest to weakest. Solve any question of Alcohols Phenols and Ethers with-. Rank the following compounds in order of decreasing basicity with 1 being the most basic and 5 being the least basic.
Acids can be reduced to aldehydes by. IIIIVIII IVIIIIII IIIIIIVI IVIIIIII IIIVIIIII For the following reaction label the acid base conjugate acid and conjugate base. Phenol 4-nitrophenol 4-aminophenol 4-bromophenol.
Ie diketone diester ketone ester. OCH3 OCH3 OCH3 NHCH3 H A B C D. Apr 082022 - Rank the following compounds in order of decreasing acidity of the indicated hydrogen i ii iii aI II IIIbIII I IIcI III IIdIII II ICorrect answer is option B.
III IV II I more nearer Br atom stronger the acid 2. The order of decreasing acidity would be. To rank items as equivalent overlap them.
II Aldehyde OC-H resonance due to pi bond. Rank the acids shown in decreasing strongest to weakest order of acidity OH CL BR. To rank items as equivalent overlap them.
Rank the following compounds in order of decreasing acid strength using periodic trends. By the -I a cid. 10 pts 1 H N OH N Li Li 2 4 3 5 6.
HI HBr MgH2 H2Se. Strongest acid ACDB weakest acid b. A CH3CHCH2 CH3CH2CH3 CH3CCH b CH3CH2CH2OH CH3CH2CO2H CH3CHClCO2H c You can easily generate random numbers in a spread-sheet that have an.
I Ether O-C-H C is lower in electronegativity than O. Group in para position causes more electron-withdrawing effect than that in meta position 1 will be less acidic than 3. Br OH OH OH Cl Decreasing acidity.
Rank the following compounds in order of decreasing acidity. Strongest acid CDBA weakest acid Answers. Conversion to the acid chloride followed by treatment with LIALH OC CH333.
Rank the following acids in order of increasing weakest to strongest acidity. A D B C. C H 3 S H I I.
This is because of the relative stability of the anions formed. H 2 O I V. List the most acidic first.
C H 3 O H I I I. Rank the acid strength from strongest to weakest. Rank the following compounds in decreasing order of expected acidity ie.
Strongest acid CDBA weakest acid Question. The decreasing order of acidic character of the following is. To rank items as equivalent overlap them.
Rank the following compounds in order of decreasing acidity putting the most acidic first. What is important here is the pka of the alpha hydrogens which are adjacent to the respective functional groups. CH4 NH3 HF H20 I II III IV Multiple Choice IV II L III IV T IV II III IV.
Without consulting the table of acid-dissociation constants match the following acids to the given Ka1 valuesA. List the compounds in each of the following groups in order of decreasing acidity. Rank the following compounds in order of decreasing acidity with 1 being the most acidic and 5 being the least acidic.
Institute of Chemical Technology. Carbonyl Compounds III. Es 9 of 19 I Review Constants Periodic Table Rank the following compounds in order of decreasing acid strength using periodic trends.
Rank the following organic compounds in order of decreasing acidity Answer. С D Selected Answer. Further presence of electron withdrawing group and its stronger effect stabilizes the phenoxide more.
Solution for Rank the following compounds in order of decreasing acidity.
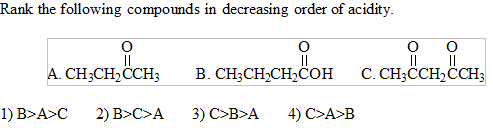
Solved Rank The Following Compounds In Decreasing Order Of Chegg Com
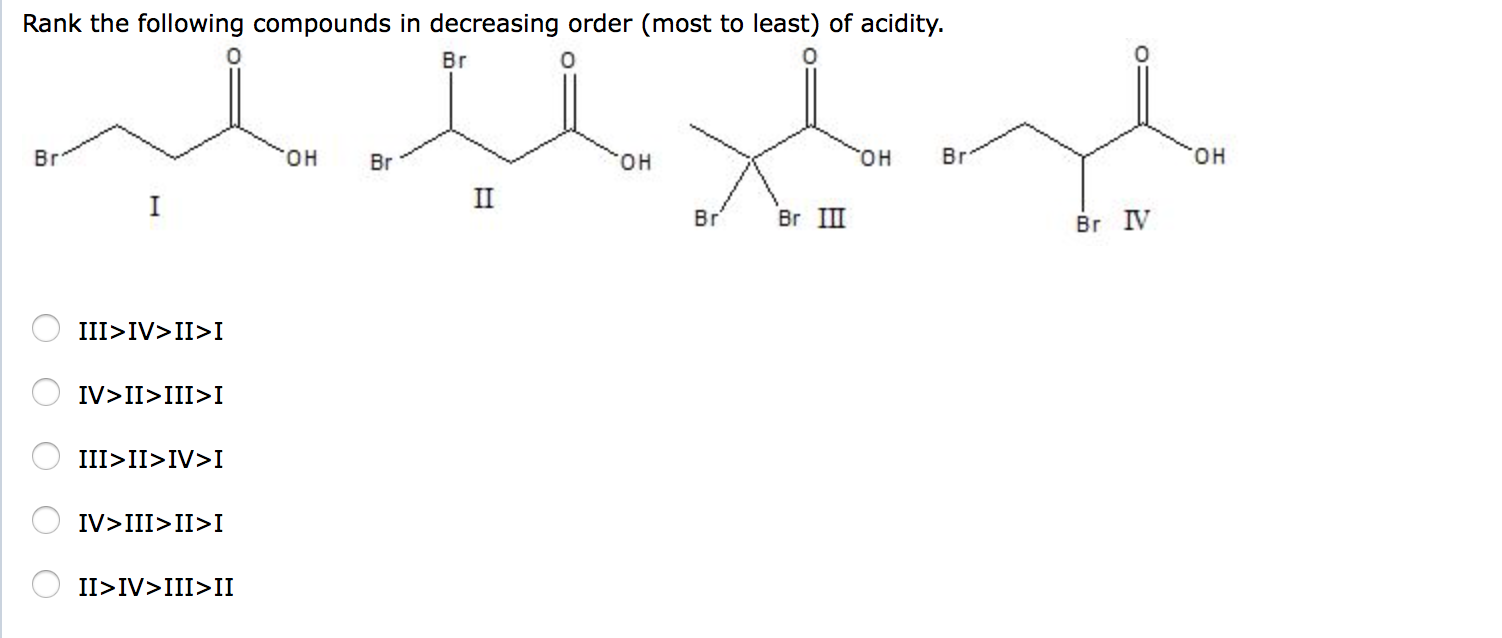
Solved Rank The Following Compounds In Decreasing Order Chegg Com
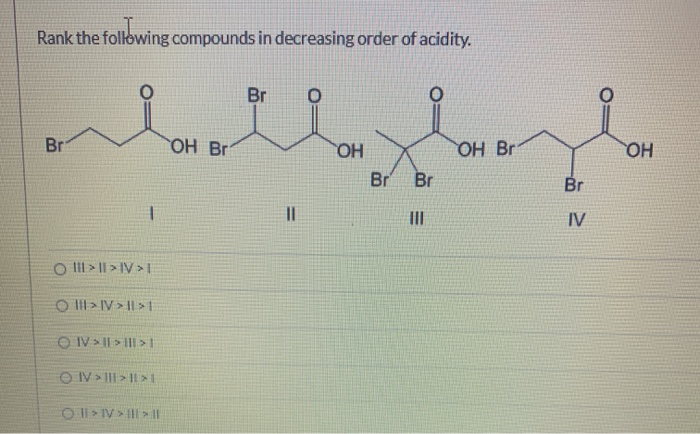
Solved Rank The Following Compounds In Decreasing Order Of Chegg Com
0 Comments